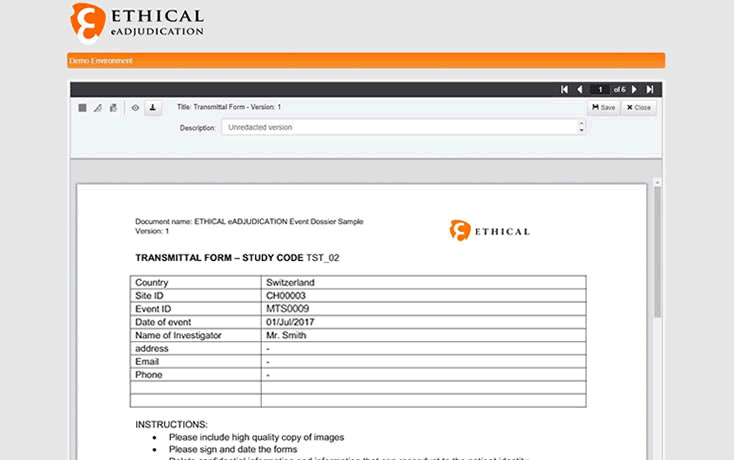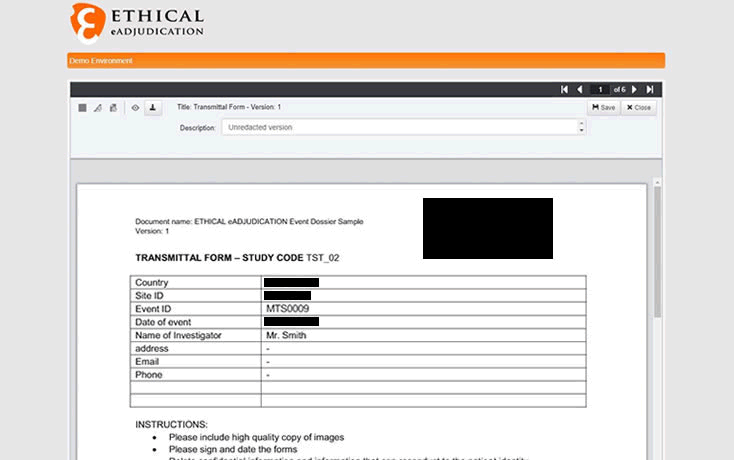
Redaction technology improves the functionality of the eAdjudication® software platform by allowing to selectively hide information on source and administrative documents.
BASEL, Switzerland, June 6, 2019,
Ethical the Swiss company providing eClinical Software Services with a special focus on Clinical Endpoint Adjudication, integrates redaction capacity to its eAdjudication® software platform to allow online anonymization of documents uploaded by investigational sites.
Source documents needed for the adjudication of clinical endpoints such as patient charts, physician’s notes or procedure reports often contain personal identifiers and other confidential information that needs to be deleted or hidden to preserve the anonymity of the subjects. In addition, adjudicators must be blinded to other information such as site number, country or investigator’s name to ensure unbiased and impartial assessment of the cases.
In the framework of endpoint adjudication site personnel anonymize source documents and reports while hiding study information is the responsibility of the sponsor’s central staff. In both cases, redaction of documents generates a heavy administrative burden.
Ethical’s eAdjudication®, the leading electronic endpoint adjudication platform, now has a new feature that allows authorized users to easily and quickly redact documents online as necessary ensuring efficacy and compliance.
“Ethical’s eAdjudication® platform continues to improve with additional capabilities such as the new redaction functionality” says Mimmo Garibbo, CEO of Ethical GmbH. “We are very proud to continue to make our customers’ job easier by allowing investigational sites and sponsors’ staff to redact documents in just a few clicks.”
About Clinical Endpoint Adjudication
The procedure by which clinical events identified as potential endpoints are submitted to a panel of independent experts to be assessed in a blinded way. Adjudication is used in drug and medical device clinical trials to manage subjective evaluations like imaging and adaptive design.
About eAdjudication®
eAdjudication®, Ethical’s electronic adjudication management system, is a cloud-based platform designed to manage clinical endpoint independent review and adjudication in a simple, effective and GxP-compliant way.
About Ethical
Founded in Basel in 2014, Ethical GmbH is an eClinical Company specialized in endpoint adjudication with a cumulative experience of 300 international clinical trials, more than 10,000 investigator sites and hundreds of thousands of patients.
DOWNLOAD NOW THE FREE ENDPOINT ADJUDICATION HANDBOOK
The Complete Manual / Reference Book (34 pages) with all the topics related to the Independent Endpoint Adjudication Committees Management